Out Of This World Tips About How To Lower Activation Energy

T = temperature in kelvin.
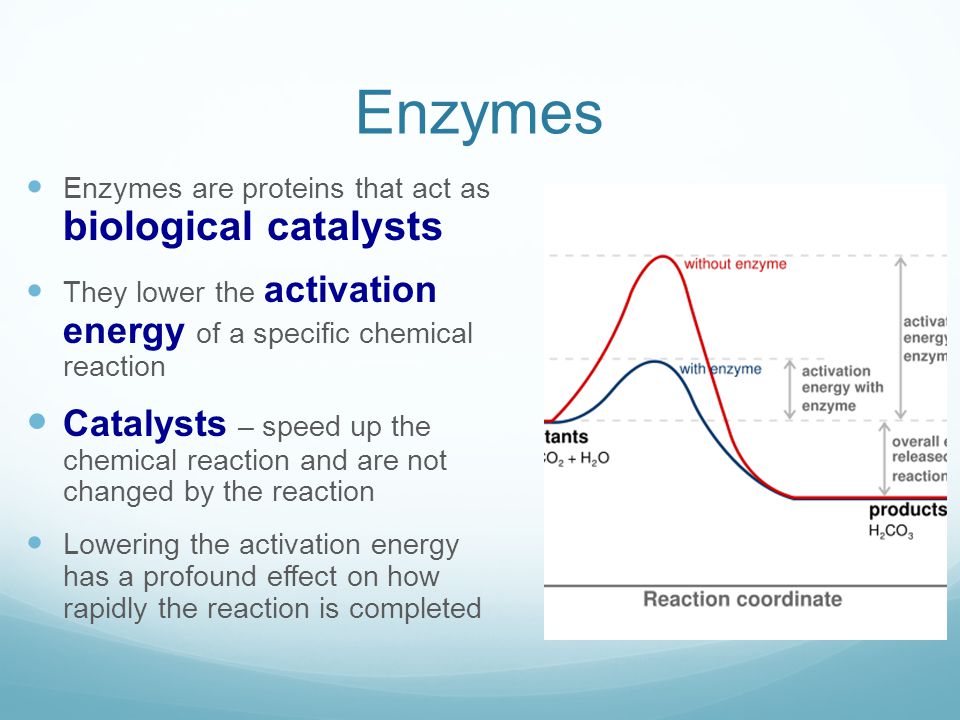
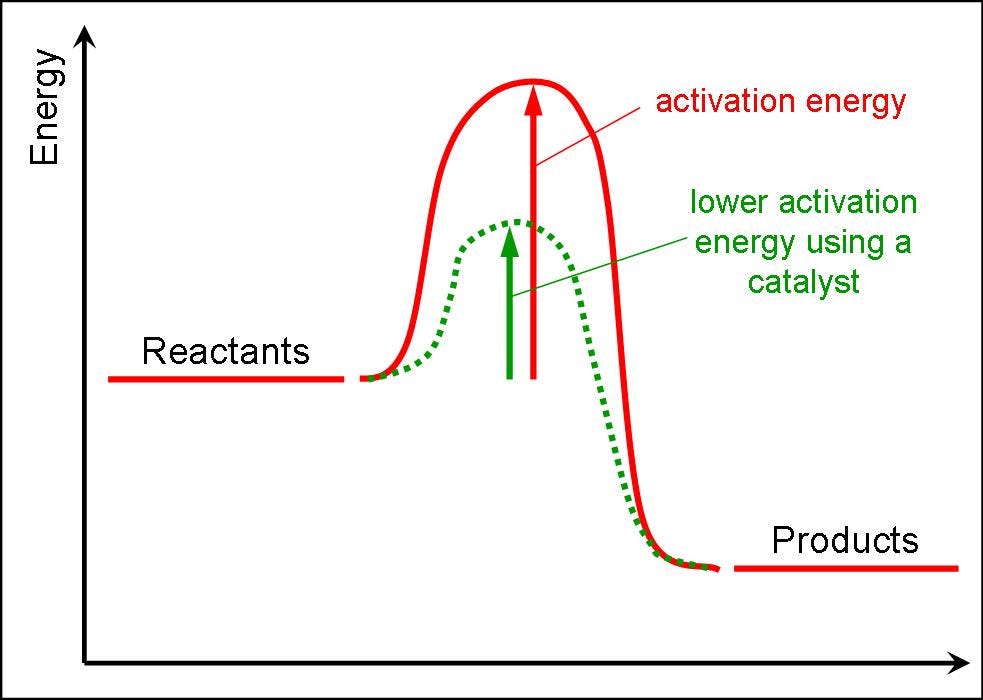
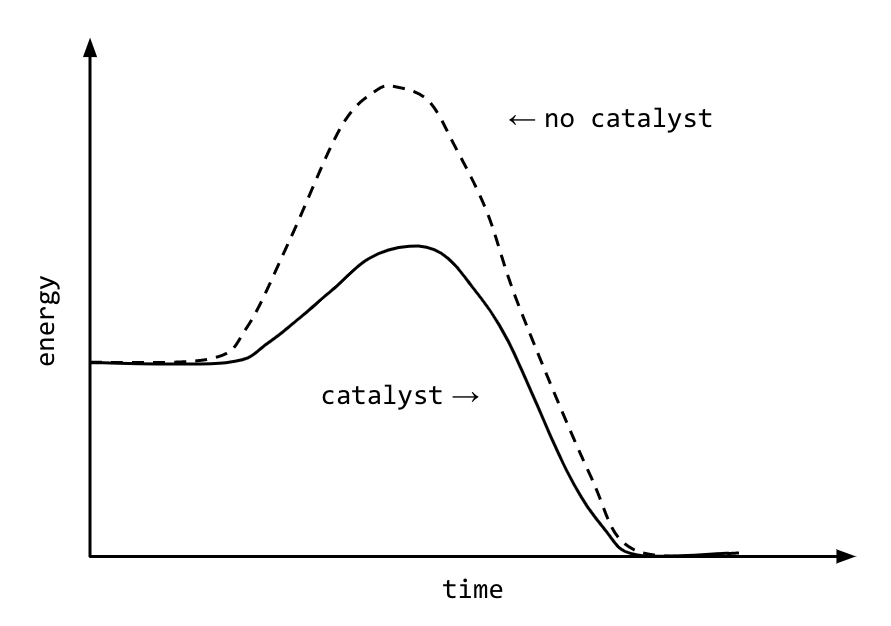
How to lower activation energy. The activation energy can be determined using the equation: Both of these molecules have properties that help to stabilize the transition state,. Catalyst remains chemically unchanged at the end of a reaction.
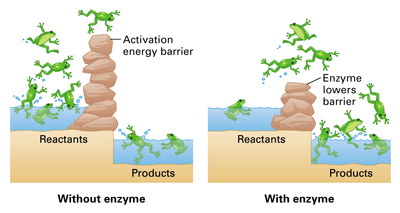
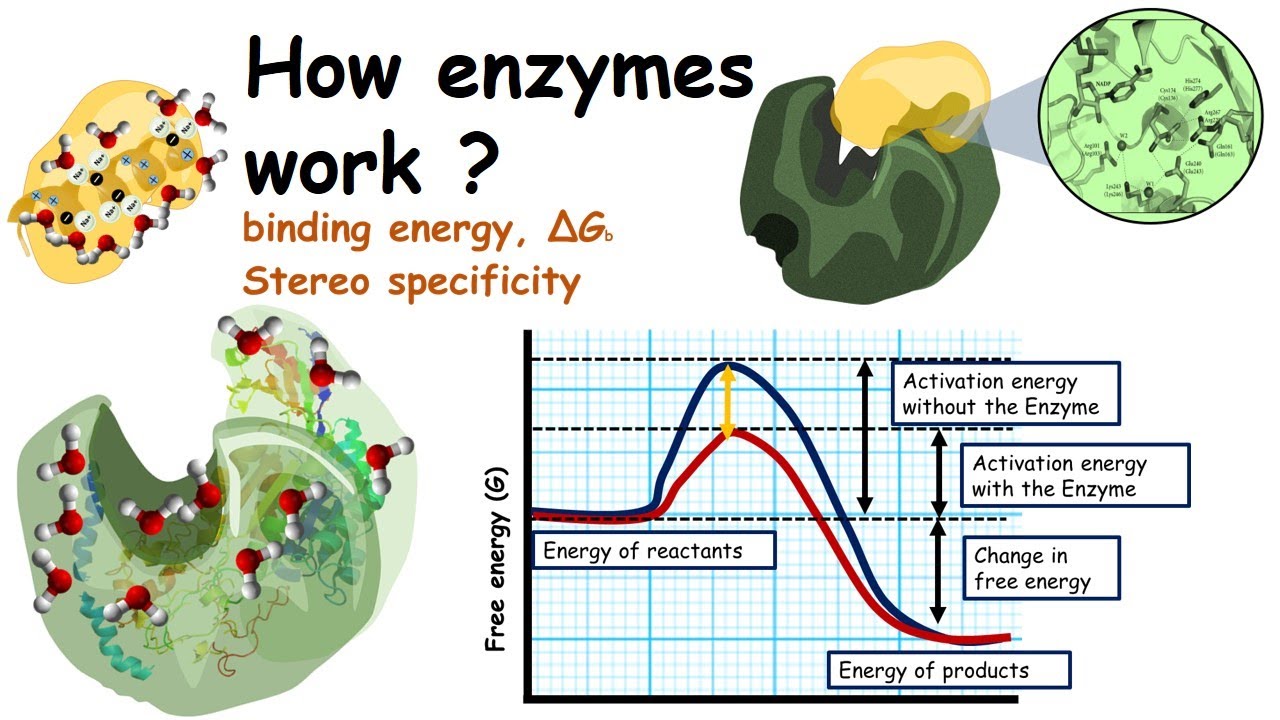
Enzymes lower activation energy through various means, including positioning substrates together in the. The first method is by helping orient the molecules or atoms in the reaction such. E a = the activation energy of the reaction in j/mol.
R = the ideal gas. Enzymes lower the activation energy for chemical reactions. But if you were trying to create a new protein that is more.
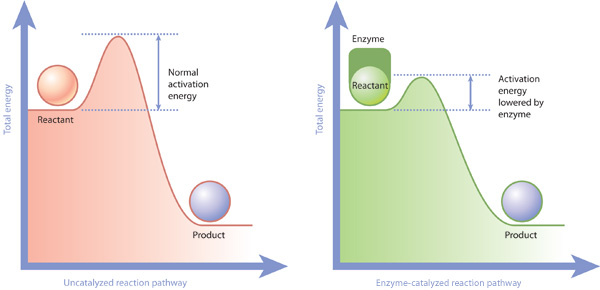
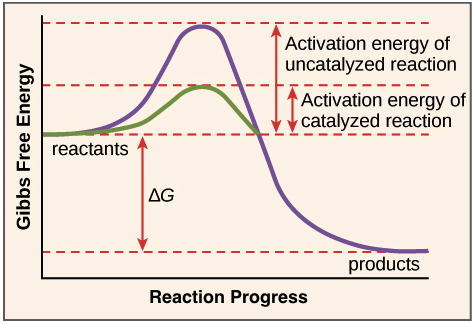
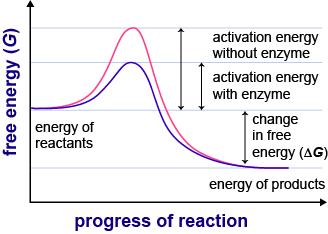
Enzymes or catalysts are two things that can be used to lower the activation energy of chemical reactions. The free energy of the reactants and products do not change,. It means that the quantity and chemical composition of the catalyst remain unchanged at the end of the reaction.
What are 4 ways enzymes can lower the activation energy of a reaction? ∴ with the increase in the activation energy e a, the rate constant k decreases, and therefore the. There are two main ways that a catalyst can lower the activation energy of a reaction.
In other words, if you are trying to lower the activation energy then you need to make it as difficult as possible. By bending substrate molecules in a way that promotes. In fact, this enzyme actually has the ability to lower activation energy, making it that much easier for your body to digest a sugar.



![Biochemistry / Activation Energy [Enzymes] - Pathwayz](https://www.pathwayz.org/Node/Image/url/aHR0cHM6Ly9pLmltZ3VyLmNvbS8ydnFKa3dwLnBuZz8x)